
Fe(III) ferric iron or Mn(IV) Reduction.
Dissimilatory Fe(III) and Mn(IV) reduction has a major influence not only on the distribution of iron and manganese, but also on the fate of a variety of other trace metals and nutrients, and it plays an important role in degradation of organic matter
Some Geobacter species have been shown to fix nitrogen.
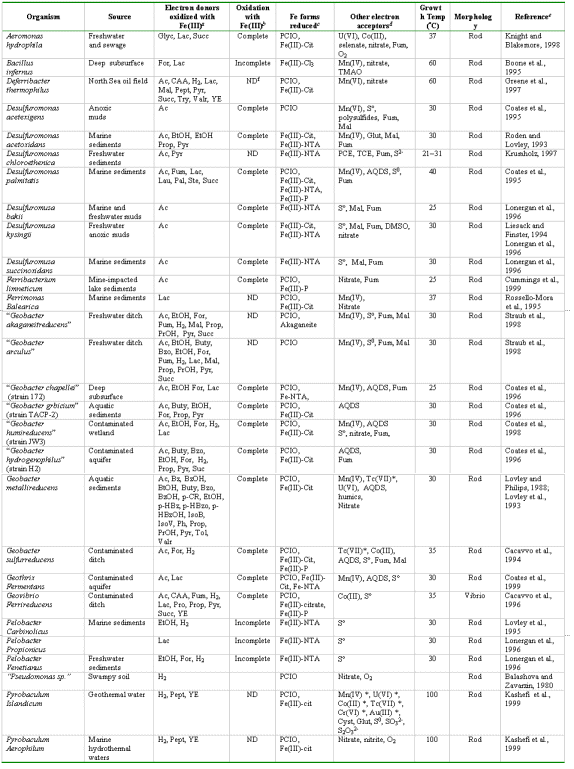
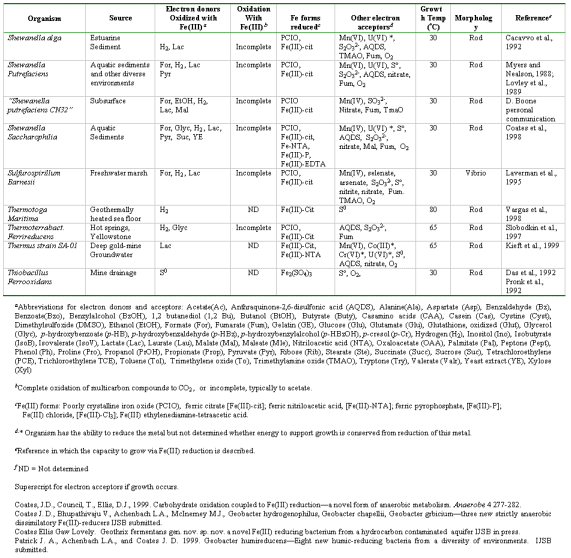
Found in anaerobic environments such as aquatic fresh and marine sediments, aquifers, deep subsurface.
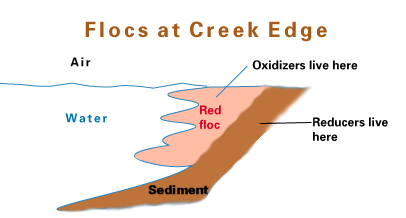
Evidence of Fe reduction is that clays will go from red-orange (presence of FeIII) to gray indicated ferrous iron production. See http://www.riversalive.org/iron_bacteria.htm. The reduction of Fe(III) produces magnetite, Fe3O4, (indicating that the reduced Fe or Mn is in solid phase as reduced minerals).
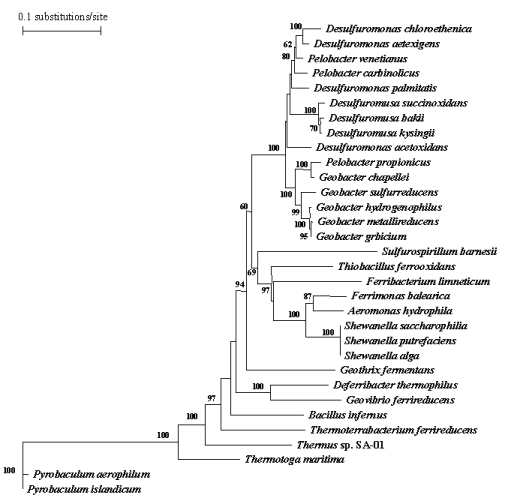
Presence of this metabolism in most all of the most deeply branching lineages in 16s tree, using H2 to reduce FE(III), allows for the speculation that it is ancient. The concept that Fe(III) reduction is an early form of respiration agrees with geological scenarios that suggest the presence of large quantities of Fe(III) on prebiotic Earth (Cairns-Smith et al., 1992; de Duve, 1995) and elevated hydrogen levels (Walker, 1980)—conditions that would be conducive to the evolution of a hydrogen-oxidizing, Fe(III)-reducing microorganism.
The magnetite produced during microbial Fe(III) reduction can be an important geological signature of this activity. For example, large quantities of magnetite at depths up to 6.7 km below the Earth's surface provided some of the first evidence for a deep, hot biosphere (Gold, 1992). Caveat: According to H Ohmoto (Personal communication), ff you ppt geothite (Fe(OH)3 then it can convert into hematite (Fe2O3) and magnetite - by passing Fe++ through Geothite. Geothite doesn’t form in temperatuare above 80-100 C, hematite forms at 220- 260 C http://news.bbc.co.uk/1/hi/sci/tech/3220633.stm
In the absence of Fe(III) or other suitable electron acceptors, some organisms in the Geobacteraceae can transfer electrons to protons to produce hydrogen gas. For hydrogen production to be thermodynamically favorable, a sink for hydrogen, such as a hydrogen-consuming microorganism, must keep hydrogen concentrations low enough. For example, several Pelobacter species can oxidize ethanol to acetate and carbon dioxide when grown in association with hydrogen-consuming microorganisms (Schink, 1992). G. sulfurreducens can oxidize acetate to carbon dioxide when cultured with Wolinella succinogenes, which oxidizes hydrogen with concomitant reduction of nitrate (Cord-Ruwisch et al., 1998).
Researchers for literature search: Derek Lovley, Ken Nealson