


Next: biometric.soc.03
Up: Biometric Society (ENAR &
Previous: biometric.soc.01
Sponsoring Section/Society: ENAR
Session Slot: 2:00- 3:50 Monday
Estimated Audience Size: 300
AudioVisual Request: Two Overheads
Session Title: Statistical Models for Longitudinal Studies: Unifying
Longitudinal and Survival Analysis Methods
Description of session: Data collected in studies in public
health routinely include both observations of times to events of interest and
other measures taken repeatedly over time; e.g. in AIDS clinical trials,
both time to death or other event and longitudinal measures of CD4 count,
viral load, and other immunological or virological variables are observed.
This session focuses on recent advances in methods of analysis that exploit
information of both types.
Theme Session: No
Applied Session: Yes
Session Organizer: Hogan, Joseph
Brown University
Address: Center for Statistical Sciences
Box G-H
Brown University
Providence RI 02912
Phone: 401-863-9243
Fax: 401-863-9182
Email: jhogan@stat.brown.edu
Session Timing: 110 minutes total (Sorry about format):
Opening Remarks by Chair - 5 minutes
First Speaker - 25 minutes
Second Speaker - 25 minutes
Third Speaker - 25 minutes
Discussant - 20 minutes
Floor Discusion - 10 minutes
Session Chair: Hogan, Joseph
Brown University
Address: Center for Statistical Sciences
Box G-H
Brown University
Providence RI 02912
Phone: 401-863-9243
Fax: 401-863-9182
Email: jhogan@stat.brown.edu
1. The Analysis of Longitudinal Data - An Expanded Perspective
Zeger, Scott,
Johns Hopkins University
Address: Department of Biostatistics
Johns Hopkins University
615 N. Wolfe Street, Room E3132
Baltimore MD 21205-2179
Phone: 410-955-3067
Fax: 410-955-0958
Email: szeger@jhsph.edu
Abstract: Longitudinal studies in public health permit observations of both
times to key health events as well as repeated measures of health outcomes.
Statistical methods for times-to-events (survival analysis)
and for repeated events (longitudinal data analysis) have
developed along independent lines even though substantive
questions can often be addressed through joint analysses of
both kinds of outcomes.
In this talk, we consider opportunities for unifying statistical
methods for times-to-events and repeated measures under an expanded
definition of longitudinal data analysis. The ideas will
be illustrated with examples from public health research.
2. Nonparametric Incomplete Data Estimation of Stochastic Process
Structures Related to Survival Models
Jewell, Nick,
University of California, Berkeley
Address: Department of Statistics
University of California at Berkeley
367 Evans Hall #3860
Berkeley CA 94720-3860
Phone: 510-642-5361
Fax: 510-642-7892
Email: jewell@stat.Berkeley.edu
Abstract: Considerable attention has been given to nonparametric
estimation of simple survival models based on data subject to random
censoring and truncation. Recently, there has been additional interest in
similar problems based on current status data. In this talk, we will
discuss extensions of some of these ideas by considering survival data in a
stochastic process setting. The extensions we have in mind are to (i)
multidimensional survival processes, and (ii) more general stochastic
processes than the familiar two-state survival models. The first of these
extensions includes consideration of so-called marker processes where one
component of the process is a simple survival counting process and other
components represent marker variables thought to be associated with
survival.
3. Non-ignorable Non-response in Longitudinal Studies with
Continuous Drop-out Times
Scharfstein, Dan,
Johns Hopkins University
Address: Department of Biostatistics
Johns Hopkins University
615 N. Wolfe Street, Room E3132
Baltimore MD 21205-2179
Phone: 410-955-3067
Fax: 410-955-0958
Email: dscharf@athena.biostat.jhsph.edu
Robins, James M.,
Harvard School of Public Health
Abstract: Consider a longitudinal study in which we follow subjects for
T months
after enrollment. At this point, we measure the primary endpoint of interest,
Y. In addition to this measurement, a series of baseline covariates,
V(0), and time dependent covariates, V(t), are collected. We are
interested in making inference about the mean of Y, 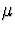 . Unfortunately,
some individuals drop-out before time T. If we let Q denote the
continuous drop-out
time, then the observable data for an individual is
. Unfortunately,
some individuals drop-out before time T. If we let Q denote the
continuous drop-out
time, then the observable data for an individual is 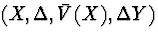 , where
, where 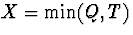 ,
, 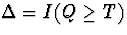 , and
, and 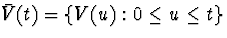 is the covariate history through time t.
If Q is unrelated to Y, then Y is said to be missing completely at random
and the sample average of the observed outcomes is an unbiased estimate of
is the covariate history through time t.
If Q is unrelated to Y, then Y is said to be missing completely at random
and the sample average of the observed outcomes is an unbiased estimate of
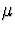 .
.
The purpose of this paper is to show how to make inferences about 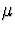 when
the drop-out time is modeled semiparametrically, no restrictions are placed on
the joint distribution of the outcome and covariates, and the outcome
is not missing completely at random. In particular, we assume that the
law of Q given
when
the drop-out time is modeled semiparametrically, no restrictions are placed on
the joint distribution of the outcome and covariates, and the outcome
is not missing completely at random. In particular, we assume that the
law of Q given 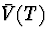 and Y follows a proportional hazards model
of the form
and Y follows a proportional hazards model
of the form

where 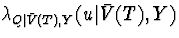 is the hazard of Q given
is the hazard of Q given
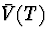 and Y,
and Y, 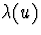 is some arbitrary, non-negative function
of u,
is some arbitrary, non-negative function
of u, 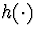 is a r-dimensional function of the covariate history,
is a r-dimensional function of the covariate history,
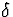 is an rx1 vector of unknown parameters, and
is an rx1 vector of unknown parameters, and 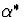 is a
known constant. Note that
is a
known constant. Note that 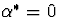 corresponds to missing at random and
corresponds to missing at random and
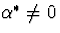 corresponds to non-ignorable non-response. We have
chosen to fix this latter constant because we know that it is at best difficult
to
estimate. Instead, we perform a sensitivity analysis
to see how inference about
corresponds to non-ignorable non-response. We have
chosen to fix this latter constant because we know that it is at best difficult
to
estimate. Instead, we perform a sensitivity analysis
to see how inference about 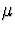 changes as we vary
changes as we vary 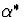 over a
plausible range of values. We apply our approach to the analysis of ACTG
175, an AIDS clinical trial in which we define the primary outcome as CD4 at 56
weeks after enrollment. Repeated measurements on CD4 counts act as the time
dependent covariates, while gender, age, race, and information on other
risk factors serve as the baseline covariates.
over a
plausible range of values. We apply our approach to the analysis of ACTG
175, an AIDS clinical trial in which we define the primary outcome as CD4 at 56
weeks after enrollment. Repeated measurements on CD4 counts act as the time
dependent covariates, while gender, age, race, and information on other
risk factors serve as the baseline covariates.
Discussant: Wei, L.J.
Harvard School of Public Health
Address: Department of Biostatistics
Harvard School of Public Health
677 Huntington Avenue
Boston MA 02115
Phone: 617-432-2826
Fax: 617-739-1781
Email: wei@hsph.harvard.edu
List
of speakers who are nonmembers: None



Next: biometric.soc.03
Up: Biometric Society (ENAR &
Previous: biometric.soc.01
David Scott
6/1/1998
![]() when
the drop-out time is modeled semiparametrically, no restrictions are placed on
the joint distribution of the outcome and covariates, and the outcome
is not missing completely at random. In particular, we assume that the
law of Q given
when
the drop-out time is modeled semiparametrically, no restrictions are placed on
the joint distribution of the outcome and covariates, and the outcome
is not missing completely at random. In particular, we assume that the
law of Q given ![]() and Y follows a proportional hazards model
of the form
and Y follows a proportional hazards model
of the form
![]()